
93. Альтернативные модели роли неосознаваемого конфликта в патогенезе психосоматической болезни. Г. Вайнер (93. Alternative Models to the Role of Unconscious Conflict in the Pathogenesis of Psychosomatic Illness. Herbert weiner)
Albert Einstein College of Medicine, USA
1. Introduction
The history of the Ideas that underlie much of research in Psychosomatic Medicine has been written many times; this paper will, therefore, recapitulate them briefly. The personality correlates of medical illness outlined by Dunbar and her coworkers (1943) can in retrospect be seen as serious attempts at a psychological classification about the predisposition to illness. However, this classificatory scheme of Dunbar made no statement either about the initiation of illness, i.e., about the role of psychological factors in the pathogenesis of disease, nor about the role of unconscious conflicts in predisposing, initiating or sustaining the disease.
It remained for Alexander (1950) to point out the role of such conflicts in disease. As is well known, he implied that: (1) they predisposed to the diseases in combination with a series of physiological, and biochemical factors that today can be traced back to genetic variation. We have also learned that immunological factors play an important role in the predisposition to some of these diseases (e.g., rheumatoid factors in rheumatoid arthritis, anticolon antibodies in ulcerative colitis, IgE antibodies in bronchial asthma). (2) Alexander clearly stated that these unconscious conflicts were activated by specific life experiences. (3) The emotional concomitants of the activated conflict were translated into physiological, that is, hormonal and autonomic outputs, in some as yet unexplained manner to initiate the disease in the predisposed.
Much controversy has surrounded the verification of the specific, but not unique, nature of these conflicts: In part, verification has been achieved (Alexander et al., 1968; Weiner et al., 1957). In the meantime, however, much American research has focused on determining the nature of the onset conditions of these illnesses. Engel and his coworkers (1967) and Parkes (1964) emphasize the role of bereavement as a general onset condition of these diseases and have detailed the nature of the psychological distress and adaptive failure bereavement occasions. Holmes and Rahe (1967) state that cumulative changes in the lives of patients correlate with the onset of many diseases, suggesting that stress consists of any event which changes the steady- state of the human organism.
These formulations seem to have considerable validity, and are not necessarily in conflict with Alexander's formulations. They emphasize social and psychological factors in disease onset but do not tell us how these factors set in motion the processes mediated by the brain which culminate in diseased organs and tissues. In part, this problem - the translation of the psychological into the physiological - bedevils all of psychosomatic research. Up to this point, most theorists in this area have assumed that man responds to totality (or Gestalt) of the perceived change in his environment. Animal experiments, however, suggest that this assumption is not correct (Hofer, 1971; Hoferand Weiner, 1971a&b, 1975; Weiner, 1972). In addition we have no idea, given the present state of our knowledge, how the brain perceives Gestalten and assigns meanings to them.
But even if we knew the answer to the question of how the nervous system perceives structures and assigns meaning to them, we would still not know how to relate such a perception and the emotional response it elicits to the output via autonomic, humoral and neuromuscular channels. Implicit in this statement is the abiding belief that the perceived event and the emotional responses it occasions give rise in a causal, linear manner to physiological responses which are controlled by the brain. The problem thus stated is that fear and joy, for example, "cause" an increased heart rate.
Specifically, how is a psychologic experience translated by the brain into a physiologic event? Or, to put it another way, how does a nonmaterial process such as the emotional response to a "stressful" situation produce material changes, such as the elevation of urinary catecholomine levels or an increase in heart rate? This particular question has baffled philosophers and scientists for many centuries, of course. Yet it remains unanswered, which would seem to indicate that our basic approach to its solution may need to be reexamined. To begin with, I would draw attention to the manner in which this question is traditionally stated: it clearly implies that there is a causal link between nonmaterial and material events. I would like to suggest two other possibilities: This causal link between psychologic events and physiologic changes may be much more complex than we have been led to believe. Or this assumed causal link may not exist at all: Concomitant events are not necessarily causally related.
We have continued to cling to the assumption that there is a causal link between these events, despite our inability to accumulate empirical data to show that in man psychologic response and physiologic change are highly correlated. Moreover, we have rationalized our failure to accumulate such data on the grounds that there are individual variations in response, or, more precisely, individual methods of coping with experience, and that these influence the physiologic response in some manner. Indeed, such individual coping responses can be identified. But this does not mean that they alone can be held responsible for the low correlation between psychologic and physiologic responses.
2. Three Classical Models of Transduction
It is the contention that one of the major reasons for our failure to solve this problem is that we have had only one hypothesis to guide us. this hypothesis is best exemplified by the fact that the emotional response is the mediator of the threatening stress and the physiological responses. This model is a linear one, although modified versions exist which suggest that the physiological responses can also attain conscious experience, presumably mediated by visceral afferents. However, the problem is again complicated by the fact that we do not know how impulses arriving over sympathetic or vagal afferent pathways can be perceived and acquire conscious meaning.
Thus, whether the line is straight or curves back on itself, we are faced repeatedly by the insuperable problem of how nerve impulses, changes in enzyme levels or turnover rates of transmitter substances (putative or actual) can "produce" ideas, thoughts, images, feelings, moods, or vice versa.
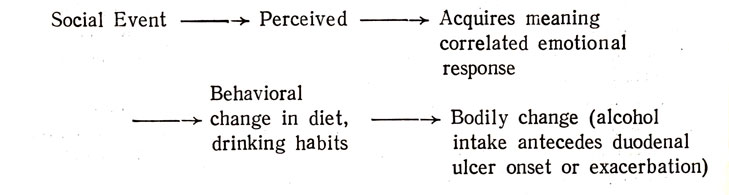
There are naturally several ways of looking at this problem. I have already mentioned the traditional linear model which most psychobiologists have employed. It is best exemplified by Fig. I taken from Knapp (1971). In it the social event or situation is perceived (1), or not, acquires emotional meaning (2) which may interact with inferred defense mechanisms which if they are effective modify and temper the physiological responses (3) which may themselves be perceived (4) or effect the social event (5) - for example, the child in an asthmatic attack may elicit a solicitous (or other) response from a parent, nurse, or a group of its peers. Further specification of this model entails the realization that acute changes in the environment may involve preparation to take action and anticipatory responses with their accompanying bodily changes, especially during novel experiences. Reactive and different responses also occur both during novel and routine experiences.
A modification of this point of view states that correlated psychological and physiological responses appear so different because they both are the product of the differences in the techniques whereby we study them. The mental experience - i.e., the thought, mental image or feeling - is the inner aspect of the subject's response to the event, as he perceives it. The neural and bodily events are the outer, and often measurable, aspects of his response to the event. The connection between the mental and the neural and/or bodily processes cannot be established by simple cross-identity. The difference between these processes (e.g., extension in space and time in the case of bodily events and nonextension in the case of mental ones) lies in the way they are presented and accessible to us. They must, therefore, be studied by different techniques, of which they are a product. The problem with this line of reasoning is that because we have used different techniques, coextensive in terms of temporal boundaries, we have assumed that if two processes (e.g. the performance of a task, and an increase in heart rate or a rise in blood pressure) occur concomitantly, they must be causally related.
In this second point of view, the psychological and the physiological are merely two different sides of a coin. However, the interpretation of how both come about is subject to misinterpretation, as stated. In addition this point; of view leaves open the question of how the two categories of events come about at all. Being two sides of the same coin they might be the product of a third variable. For example, the behavioral and psychological changes seen in primary anorexia nervosa, and the correlated immaturity of circadian pattern of luteinizing hormone secretion, may simply be the "read out" of a defect in the anterior hypothalamus.
The third and classical point of view is that despite the coextensiveness of the psychological and physiological, the two belong to wholly unrelated realms of events. Although we may study the physiology and psychology of an ill person concomitantly, they really have nothing to do with each other and proceed independently. In this view a bereavement may produce grief in the bereaved, but the physiological changes of weeping, or of rheumatoid arthritis, are independent of his loss. In this view, the physiological change arises in no understandable manner.
3. An External Loop Model of Transduction
In a very serious way, then, our failure to solve this central and time-honored empirical and conceptual issue may be due to the fact that there is no solution to it because the problem is incorrectly assumed to be a real one.
There is a model which I have termed the "external loop" model. Briefly stated it is that in some instances the initiating social event, perceived as a threat, is not coped with, and produces emotional responses which are correlated with changes in behavior. It is well-known that some patients who become anxious may medicate themselves or obtain medication from physicians. Other patients who become depressed either gorge themselves, do not eat, or drink, alcohol. In Weisman's series (1956) exacerbations of duodenal ulcer occurred in various settings, with various emotional and psychological reactions leading to the drinking of alcohol, a known antecedent of duodenal ulcer exacerbations.
Similarly, in a recently completed study Katz and his coworkers (1973) have observed that in 60% of patients' attacks of gout followed situations in which predisposed patients had to prove themselves, and which lead them to eat and drink alcohol in excess of the test of their occupational prowess.
In other words, given a particular predisposition, some persons when faced with a particular situation will respond not only psychologically but also behaviorally in terms of their diet and alcohol intake. More recently, also, it has been demonstrated that patients with hypertension seem to prefer diets high in salt if given a choice. We do not know whether this preference antecedes or is a consequence of hypertensive illness.
In any case, these examples suggest that in at least some cases the psychological responses to situations are mediated through changes in diet, in eating and drinking behavior in the predisposed. Heuristically, this hypothesis would be easily testable; the data could also be more reliably established and quantitated.
A variant of this model could also help us conceive of some cases of primary anorexia nervosa. It is known that about one-third to one-half (or more) of all cases of this illness begin with amenorrhoea. Evidence is now beginning to accumulate that the amenorrhoea is correlated with immature (age-inappropriate) secretory patterns of pituitary luteinizing and follicle stimulating hormone which in part may account for the amenorrhoea (Boyar et al., 1974). It is not known, however, whether these immature secretory patterns antecede the illness, or why they occur at all.
In any case the amenorrhoea antecedes the remorseless dieting which the patient with anorexia nervosa engages in. Thus, it is possible that rather than oral impregnation fantasies being the antecedents of the illness in some cases; they may be the consequences of the amenorrhoea. To be more explicit: the amen, orrhoea may be subject to (mis)interpretation by the patient as a pregnancy. The patient explains the cause of the pregnancy to herself as deriving from conflictful fantasies of oral impregnation, which in turn lead to not eating.
Thus we have two variants of the "external loop" model which can be represented in the following manner:
External Loop Model A
Clearly, the applicability of Model В to some forms of anorexia nervosa is subject to empirical test. It is not the sole explanatory model in all instances of this illness. For example, it would not apply to those instances in which amenorrhoea is a late manifestation of the illness which begins with not eating and weight loss.
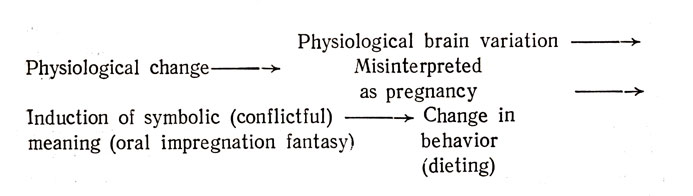
External Loop Model В
Some authorities believe that "true" or "primary" anorexia nervosa is not initially a disturbance of eating but is characterized by a failure to recognize enteroreceptive hunger signals and to be able to discriminate these from other bodily sensations and feelings. In addition, the burden on this failure is placed on early experiences during the feeding of the infant, so that either the sensation of hunger or its meaning is never learned (Bruch, 1973). In addition, the child is a very compliant and model one, and has at the onset of puberty a great fear of growing fat and therefore does not eat. Some experts do not believe that anorexia nervosa is primarily a disturbance of eating behavior, although other forms of such disturbances do occur with "secondary" anorexia.
Yet there is one striking fact which this account does not consider: "true" anorexia nervosa occurs about ten times as frequently in girls as boys. When it occurs in boys it precedes puberty; in girls it follows puberty. This fact suggests a "protective" role for male sex hormones and/or an initiating role for female hormones, both controlled by their respective gonadotrophic hormones. There is much to suggest that during puberty (the age at which the incidence of some forms of anorexia nervosa is highest) a reorganization of hypothalamic function and the hypothalamic-pituitary axis occurs. In normal adolescence, there may be bouts of asceticism and self-denial during which all gratification, including eating, is suppressed, followed by bouts of self-indulgence.
Should this hypothesis be proven one could see that patients with anorexia nervosa are representative of one end of the distribution of adolescence. The behavioral, psychological, and physiological events in anorexia may be seen as a manifestation of the aberrant reorganization of hypothalamic function during adolescence manifested by tonic inhibition of eating behavior, increased motoric activity, and failure of adult patterns of LH and FSH release to I occur, although other pituitary hormones such as TSH and possibly HGH may be normally produced and/or released. The antecedents of this disturbance may well be life experience which may alter hypothalamic function, specifically levels of neurotransmitter synthesis, release, re-uptake, or degradation. In this view, it is early experience which alters bodily function and the psychology of the child. The burden of the inception of the illness is not only placed on conflictful fantasies but on a complex interplay of experience, physiology, and psychology in which the conflictful fantasy may in some instances be induced or elicited by aberrant sexual functioning producing amenorrhoea.
4. Collateral Models of Transduction
If we are to understand how the brain translates experience, the following factors must be taken into account: First, psychologic experiences may be associated with specific changes in a wide range of physiologic functions and also with exquisitely discrete and selective change at times. Second, when a variety of functions are studied during and after an experience, a pattern of change can be observed over time. Putting it another way, there is evidence that the brain has the capacity to amplify a signal, stimulus or event in space and time. But the way this pattern is regulated and amplification occurs is not fully understood. Third, previous experiences produce changes in the nervous system which not only alter the response to later experience but also change this physiologic pattern. Finally, in view of the correlation between the steady-state activity of the nervous system with behavioral steady states, it is also essential to our understanding of the process of transduction to take into account the behavioral state of the animal or human subject at the time the experience occurs.
First I would like to suggest that further insight into the events in the brain which link a psychologic experience with a series of physiologic events cannot be achieved until there has been careful analysis of the specific constellation of stimuli or events which impinge on the organism. For example, we need to know what it is about restraint-immobilization or crowding that is stressful to an animal. Is it the continuous sensory input from skin when it is closely confined, or the sensory input from muscle-tendon receptors when it struggles? Is it the deprivation of food, water, or a fall in body temperature, etc.?
Second, we need to know whether each variable is equally capable of producing the same behavioral and physiologic effect. If so, are these effects mediated by the same or by different circuits in the brain? Is it possible that one aspect of the "stressful" configuration produces the behavioral effect and another aspect the physiologic one? Some test of this speculation might be made by systematically varying these aspects.
In many ways, this line of reasoning is without precedent. As noted earlier, Mason sought the common element in his avoidance conditioning experiments which he identified as the psychologic (emotional) responses of his animals to the experimental procedure. These responses were then translated into hormonal changes - somehow. But how?
In contrast, a series of experiments described below were not only not predicated on this line of reasoning but have enhanced our understanding of the translational process. In so doing, they have provided us with models of this process which make it necessary to change our traditional view of some aspects of the mind-body relationship.
These experiments suggest that a specific stimulus to receptors simultaneously entrain more than one neural circuit. Thus, the same stimulus may somehow lead to its being experienced psychologically as light (or some other modality), or a complex visual percept via one circuit and to a non-experienced physiological change by other circuits. Clearly, the psychological experience of light does not cause the physiological change; they occur concomitantly but independently of each other. Do such collateral processes occur in Nature?
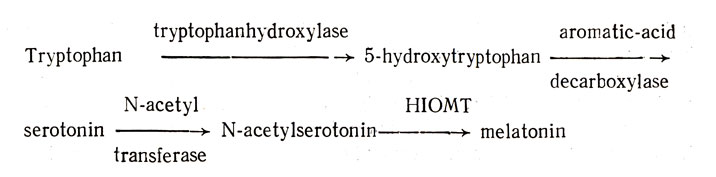
One process in which the entire transduction of input and the resulting physiologic changes are known provides a clear-cut answer to this question. I refer to the manner in which light affects the functioning of the pineal gland. Fiske et al. (1960) have shown that the weight of the pineal gland decreases when rats are continuously exposed to light and that female rats remain in continuous vaginal estrus under such lighting conditions (Browman, 1937; Fiske et al-, 1960). These findings plus the observation that extracts of the pineal gland of cattle inhibit estrus led Wurtman et al. (1963a) to conclude that melatonin reduces the incidence of estrus in the rat. We are further indebted to Axelrod and his group for working out the biosyn thetic pathway of melatonin from tryptophan:
More specifically, Wurtman et al. (1963b) found that levels of hydroxy-indole-O-methyltransferase (HIOMT) in the pineal gland were elevated when rats were kept in the dark and reduced when they were exposed to continuous light. The fact that light inhibits the synthesis and release of melatonin would explain why continuous light produced persistent estrus.
If we were to adhere to the hypothetic psychophysiologic model traditionally used in our field, we would say that it is the experience of light that regulates the release of melatonin (Figure 1). But this is not what seems to happen. In fact, we are now presented with a most interesting model of how an external stimulus (i.e., light) is transduced by the nervous system. Moreover, it would be premature to minimize the significance of this model on the grounds it is the one and only model that can exist. It should be noted, however, that Axelrod's work on the pineal rests on his identification of light as the critical variable, which served as a point of departure for further analysis.
Axelrod and his associates have also worked out the rather complex and indirect pathway from the retina to the pineal gland and the manner in which the biosynthetic machinery of the gland is influenced (Moore et al., 1967). As shown in Figure 2, in the mammal environmental light passes via the retina -> to the inferior accessory optic tract to the preganglionic sympathetic fibers f the spinal cord -> to the superior cervical ganglion, from which postganglionic fibers pass upward to the parenchymal cells of the pineal gland, whose terminals release norepinephrine (Axelrod et al., 1969; Axelrod, 1971).
Obviously, light also stimulates the retina to entrain impulses which pass via the classic visual pathways to the visual cortex, and, in a way which is not understood, produce the experience of light. It needs to be emphasized, however, that this experience is subserved by quite separate mechanisms from those which influence pineal functioning.
The release of norepinephrine in the pineal gland influences the formation of melatonin from tryptophan by inducing the enzyme N-acetyltransferase (Klein and Weiler, 1970) which converts serotonin into N-acetylserotonin. Two additional points about the transduction of experience and the regulation of pineal gland functioning need to be made: I would emphasize taking into account {a) the time during a biologic rhythm at which a stimulus is provided or an experience occurs, and (b) the animal's age when the experience occurs.
The significance of the first variable relates to the fact that there is a biologic rhythm for the content of serotonin (Owman, 1965) and norepinephrine in the pineal gland. The content of serotonin is high during the day under normal lighting conditions and low at 11:00 PM (Quay, 1963). This rhythm is endogenous (Snyder et al., 1965) although its driving oscillator is unknown; however, the oscillator can be entrained by light - e. g., when day and night are reversed experimentally (Snyder et al., 1967). On the other hand, norepinephrine content, which reaches its highest levels at night, is not controlled by an endogenous oscillator but is under the direct environmental control of light. Thus, its high nocturnal content corresponds to the high nocturnal content of HIOMT and therefore of melatonin synthesis.
Of particular interest, however, is the fact that the oscillator for serotonin is not operative until the rat is 6 days old. This underscores the importance of the second variable - i.e., the animal's age at the time an experience occurs. In young rats not yet 27 days old, light travels via an extraretinal pathway to affect the serotonin level in the pineal gland (Axelrod, 1971b; Zweig et al., 1966); once they have reached the age of 27 days, this earlier pathway is no longer operative and is apparently replaced by the one illustrated in Figure 3.
The principle illustrated by Axelrod's work is not unique. When the stimulus for a behavior has been clearly identified and the behavior has been carefully analyzed, one finds that even the components of the behavior, although they appear to form an integrated whole, are actually separate and distinct and, as such, merit individual analysis. Furthermore, these separate components may be subserved by different neural pathways. For example, a light touch of the dorsum of the paw of a cat produced the contact-placing reaction. However, careful analysis of this behavior discloses that the bending of hairs entrains impulses which travel by at least two routes. As illustrated in Figure 3, the early components of the biceps (EMG) and the first phase of the placing movement are activated by the ventroposterior nucleus (VP) of the thalamus. Later components are probably activated by a complex circuit which passes through VP, sensorimotor cortex, pyramidal tract, red nucleus, interpositus nucleus of the cerebellum, the ventrolateral-ventroanterior nucleus of the thalamus, and, once again, the sensorimotor cortex (Amassian et al., 1972a and b). What is more, these circuits are not fixed. A lesion in one circuit may cause the behavior to disappear for a time, only to return when, presumably, another circuit has taken over the function of the circuit which is incapable of activating the necessary behavioral components. Typically, circuit diagrams such as those provided in Figure 3 do not tell us that the information at each relay nucleus is transformed in a different manner - another aspect of the transduction process.
In summary, I think we can conclude from the available evidence that even a simple behavior, produced by a very simple stimulus, is the product of separate components which are regulated by different neural circuits. It would appear, therefore, that the critical element in the production of a behavior is the timing of the output of various components by different circuits - in the case of the contact-placing reaction, the sequential and orderly activation of different muscles of the forelimb, long after the stimulus which initiated the behavior has ceased.
Axelrod's work on the pineal gland, which gives a complete picture of the manner and mechanism by which an environmental influence on an organism is transduced into a highly relevant physiologic chain of events, appears to have broad implications for the mind-brain-body problem. It suggests that a specified input (light) may have physiologic and psychologic effects, which, though correlated and concomitant, are not causally related. In fact, the input to the receptor travels quite different neural routes to produce a complex phenomenon (the experience of light) and other behavioral and physiologic effects (a reduction in the synthesis and release of melatonin, and estrus).
As pointed out earlier, those who subscribe to the traditional model of psychophysiologic relationships would contend, in this instance, that it is the experience of light that regulates the release of melatonin. For the assumption that mental experiences initiate physiologic events is, of course, a fundamental precept of psychosomatic medicine. In light of Axelrod's findings, however, one must question the accuracy of this view of the supremacy of the mental over the physiologic.
As noted above, Axelrod's findings cannot be arbitrarily dismissed on the grounds that their application is limited to the functioning of the pineal gland. On the other hand, it is impossible to say how many instances of the model outlined occur in nature. One thing is clear, however: Axelrod's work provides us with a model which refutes the traditional, linear, theoretical model of mind-body interaction which we have accepted without reservation in psychobiology and on which we have based our research efforts to date. Thus, at the very least, Axelrod's work raises the possibility that other models could be used.
5. An Internal Loop Model
A sixth model has been proposed by Reiser (1975). In this model the inability to cope with the threat of the perceived "stress", the reactivation of the (unconscious) conflict which constitutes that person's psychological sensitivity, is associated with emotional arousal and a spectrum of autonomic and hormonal responses which in turn alter brain function, and have been shown to affect psychological functioning. For example, cognitive processes (Pollin and Goldin, 1961) and proneness to anxiety (Levitt et al., 1963) have been induced by the infusion of hydrocortisone (Weiner et al., 1963) or epinephrine; peripheral autonomic changes may also affect cognition (Callaway and Thompson, 1953). Glucocorticoids may affect catecholamine (Maas and Mednieks, 1971) and indoleamine (Azmitia and McEwen, 1969; Green and Curzon, 1968) levels in the brain and induce changes in patterns of neuronal discharge. Prednisone, for example, markedly reduced the percentage of REM sleep, an effect that is dose dependent (Gillin et al., 1972).
Reiser goes on to state that these physiological changes acting upon the brain are reflected in altered psychological functioning expressed in ever more primitive ways of perceiving and thinking, evaluating danger, and coping with it and with the unconscious conflict. In this vicious cycle of events, a consequent series of more vigorous bodily changes would next occur. He goes on to say that as these continue, the function of the brain exposed to continuing changes in circulating hormones would become more plastic - inactive brain circuits would be brought into play and "make connection with appropriate efferent fibers to the viscera" to produce altered function. The psychological expression of the activation of these pathways, he believes, is expressed in the activation of new outflow pathways so as to alter visceral function which interacts with the predispositions mentioned earlier to activate illness.
The internal loop of this model is Step I in Figure 4. It is a model which has much to commend it as it puts into order very many known facts, such as that exacerbations of many illnesses do occur during sleep, and that rhythmic processes such as circadian rhythms must be taken into consideration in understanding the pathogenesis of illness.
However, the model leaves open how being alarmed occasions physiological mobilization, or how altered brain physiology is expressed psychologically. The problem of translation of these two categories of events into each other remains unspecified, because at the present time we have no way of specifying it.
Reiser also postulated that once the outflow channels (e. g., autonomic, humoral, and neuromuscular) were activated they would alter visceral function and would interact with the predisposing factors of that particular illness. The initiating psychoneuro-endocrine sequence which he has outlined is not specific to the illness (in contrast to Alexander's hypotheses) but the predis-position to a particular illness is specific.
The model outlined certainly incorporates many known facts. All models which have been discussed in this section attempt to bridge the gap between environmental events, mental processes, brain and physiological mechanisms, and illness. Their value is heuristic: For the sake of progress in psychosomatic medicine, it may be well to have alternative ways of organizing data until our knowledge is such that a correct model will emerge which will bridge the gap between mind, brain, and body in health and illness - a gap which remains a crucial one in biology and medicine.
References
1. Dunbar, H. F. Psychosomatic Diagnosis. New York: Hoeber, 1943.
2. Alexander, F. Psychosomatic Medicine. New York: Norton, 1950.
3. Alexander, F., French, T., and Pollock, G. H. Psychosomatic Specificity. Chicago: University of Chicago y7Press, 1968.
4. Weiner, H., Thaler, M., REISER, M. F., and Mirsky, I. A. Etiology of duodenal ulcer. I. Relation of specific psychological characteristics to rate of gastric secretion (serum pepsinogen). Psychosomatic Medicine 19:1, 1957.
5. Engel, G. L. A psychological setting of somatic disease: The 'Giving Up-Given Up' Complex. Proceedings of the Royal Society of Medicine 60: 553, 1967.
6. Parkes, С. M. Recent bereavement as a cause of mental illness. British Journal of Psychiatry 110:198, 1964.
7. Holmes, T. H., and Rahe, R. H. The social readjustment rating scale. Journal of Psychiatric Research 11: 213, 1967.
8. Hofer, M. A. Regulation of cardiac rate by nutritional factor in young rats. Science 172: 1039, 1971.
9. Hofer, M. A., and Weiner, H. The development and mechanisms of cardiorespiratory responses to maternal deprivation in rat pups. Psychosomatic Medicine 33:353, 1971a.
10. Hofer, M. A., and Weiner, H. Physiologiealand behavioral regulation by nutritional intake during early development of the laboratory rat. Psychosomatic Medicine 33:468,1971b.
11. Hofer, M. A., and Weiner, H. Physiological mechanisms for cardiac control by nutritional intake after maternal separation in the young rat. Psychosomatic Medicine 37:8, 1975.
12. Knapp, P. H. Revolution, relevance and psychosomatic medicine: Where the light is not. Psychosomatic Medicine 33, 363, 1971.
13. Weisman, A. A study of the psychodynamics of duodenal ulcer exacerbations with special reference to treatment and the problem of specificity. Psychosomatic Medicine 18: 2, 1956.
14. Katz, J. L., Weiner, H., Gutman, A., and YU, T. -F. Hyperuricemia, gout and the executive suite. Journal of the American Medical Association 224:1251, 1973.
15. Boyar, R. M., Katz, J. L., Finkelstein, J. W., Kapen, S., Weiner, H., Weitzman, E. D., and Hellman, L. Anorexia nervosa: Immaturity of the 24-hour luteinizing hormone secretory pattern. New England Journal of Medicine 291: 861, 1974.
16. Weiner, H. Some comments on the transduction of experience by the brain. Psychosomatic Medicine 34:355, 1972.
17. Bruch, H. Eating Disorders-Obesity, Anorexia Nervosa and the Person Within. New York: Basic Books, 1973.
18. Fiske, V. M., Bryant, G. K., and Putnam, J. Effect of light on the weight of the pineal in the rat. Endocrinology 66:489, 1960.
19. Browman, L. P. Light in its relation to activity and estrous rhythm in the albino rat. Journal of Experimental Zoology 75: 375, 1937.
20. Wurtman, R. J., Axelrod J., and Chu, E. W. Melatonin, a pineal substance: Effect on rat ovary. Science 141:277, 1963a.
21. Wurtman, R. J., Axelrod, J., and Phillips, L. S. Melatonin synthesis in the pineal gland: Control by light. Science 142: 1071, 1963b.
22. Moore, R. Y., Heller, A., Wurtman, R. J., and Axelrod, J. Visual pathway mediating pineal response to environmental light. Science 155:220, 1967.
23. Axelrod, J., Shein, H. M., and Wurtman, R.J. Stimulation of C14 melatonin synthesis from C14-tryptophan by noradrenaline in rat pineal in organ culture. Proceedings of the National Academy of Sciences, U.S.A. 62: 544, 1969.
24. Axelrod, J. Noradrenaline: Fate and control of its biosynthesis. Science 173:598, 1971.
25. Klein, D. C., and Weller, J. Serotonin N-acetyl transferase activity is stimulated by norepinephrine and dibutvryl cyclic adenosine monophosphate. Federation Proceedings 29:615, 1970.
26. Owman, С. H. Localization of neuronal and parenchymal monoamines under normal and experimental conditions in the mammalian pineal gland. Progress in Brain Research 10:423, 1965.
27. Quay, W. B. Circadian rhythm in rat pineal serotonin and its modification by estru s cycle and photoperiod. General and Comparative Endocrinology 3:473, 1963.
28. Snyder, S. H., Zweig, M., Axelrod, J., and Fischer, J. F. Control of the circadian rhythm in serotonin content of the rat pineal gland. Proceedings of the National Academy of Sciences, U.S.A. 53:301, 1965.
29. Snyder, S. H., Zweig, M., and Axelrod, J. Circadian rhythm in the serotonin content of the rat pineal gland: Regulating factors. Journal of Pharmacology and Experimental Therapeutics 158:206, 1967.
30. Zweig, M., Snyder, H. M., and Axelrod, J. Evidence for a non-retinal pathway of light to the pineal gland of newborn rats. Proceedings of the National Academy of Sciences, U.S.A. 56=515, 1966.
31. Amassian, V. E., Weiner, H., and Rosenblum, M. Neural systems subserving the tactile placing reaction: A model for the study of higher level control of movement. Brain Research 40:171, 1972a.
32. Amassian, V. E., Ross, R., Wertenbaker, С. and Weiner, H. Cerebellcthalamocortical interrelations in contact placing and other movements in cats. In: T. Frigyesi, E. Rinvik and M. D. Yahr (Eds.) Corticothalamic Projections and Sensorimotor Activities. New York: Raven Press, p. 395, 1972b.
33. Reiser, M. F. Changing theoretical concepts in psychosomatic medicine. In: M. F. Reiser (Ed.) American Handbook of Psychiatry, Vol. 4, New York,: Basic Books.
34. Pollin, W., and Goldin, S. The physiological effects of intravenously administered epinephrine and its metabolism in normal and schizophrenic men. II. Journal of Psychiatric Research 1:50, 1961.
35. Levitt, E. E., Persky, H., Brady, J. P., and Fitzgerald, J. A. The effect of hydrocortisone infusion in hypnotically induced anxiety. Psychosomatic Medicine 25: 158, 1963.
36. Weiner, S., Dorman, D., Persky, H., Stack, T. W., Martin, J., and Levitt, E. E. Effects on anxiety of increasing the plasma hydrocortisone level. Psychosomatic Medicine 25:69, 1963.
37. Callaway, E., and Thompson, S. V. Sympathetic activity and perception. Psychosomatic Medicine 15:433, 1953.
38. Maas, J. W., and Mednieks, M. Hydrocortisone-mediated increase of norepinephrine uptake by brain slices. Science 17Ы78, 1971.
39. Azmitia, E. C., Jr., and McEwen, B. S. Corticosterone regulation of tryptophan hydroxylase in midbrain of rat. Science 166: 1274, 1969.
40. Green, A. R., and Curzon, G. Decrease of 5-hydroxytryptamine in the brain provoked by hydrocortisone and its prevention by allopurinol. Nature (London) 220: 1095. 1968.
41. Gillin, J. C., Jacobs, L. S., Fram, D. H., and SNYDER, F. Acute effect of a glucocorticoid on normal human sleep. Nature (London) 237:398, 1972.
|
ПОИСК:
|
© PSYCHOLOGYLIB.RU, 2001-2021
При копировании материалов проекта обязательно ставить активную ссылку на страницу источник:
http://psychologylib.ru/ 'Библиотека по психологии'
При копировании материалов проекта обязательно ставить активную ссылку на страницу источник:
http://psychologylib.ru/ 'Библиотека по психологии'